ODYSSEY Polar Bonds- Molecules
How do electronegativity differences affect the type of bonding in chemical compounds? Why do some (but not all) molecules have dipole moments? If a molecule has polar bonds, does that mean it is a polar molecule?
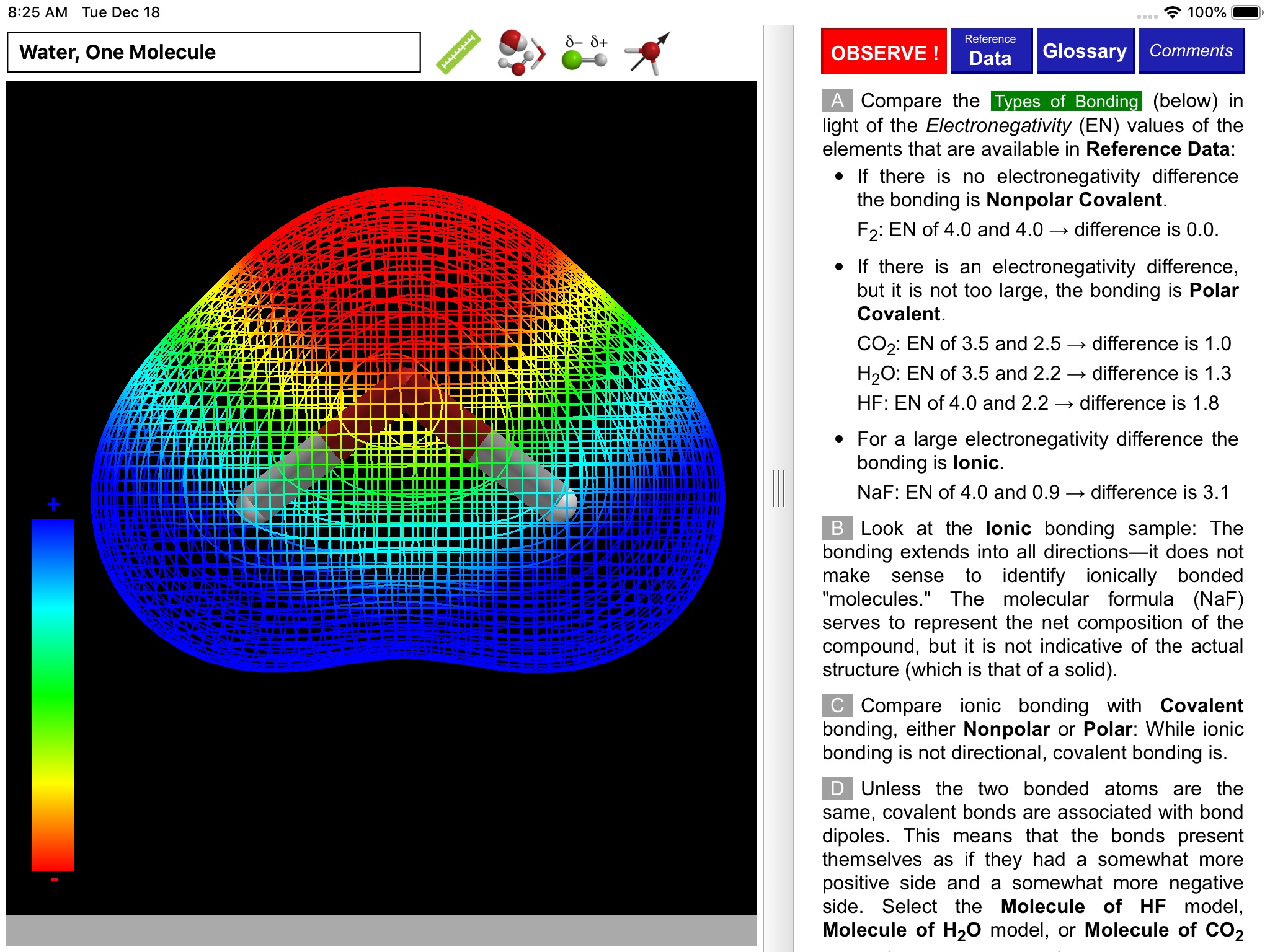
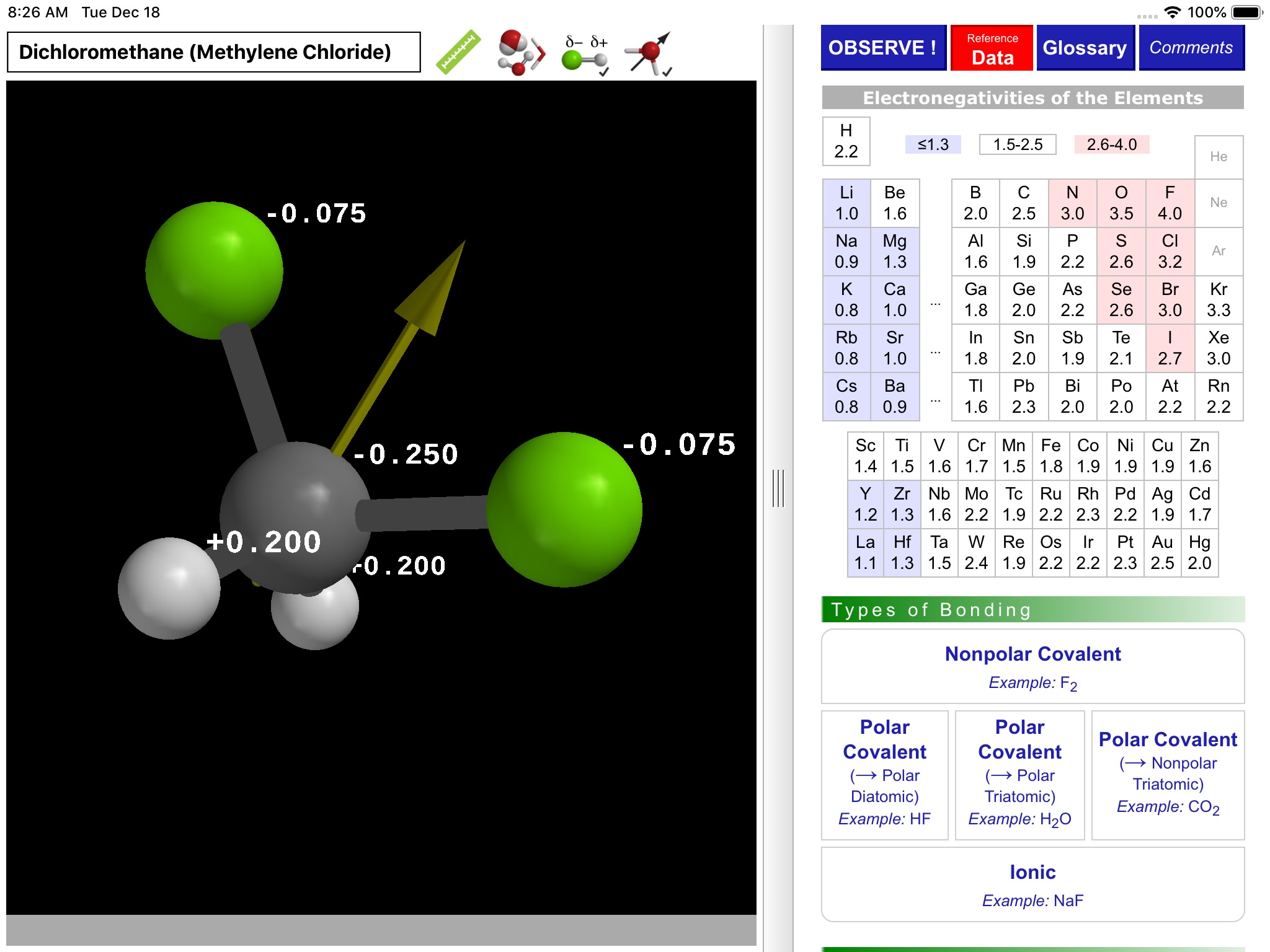
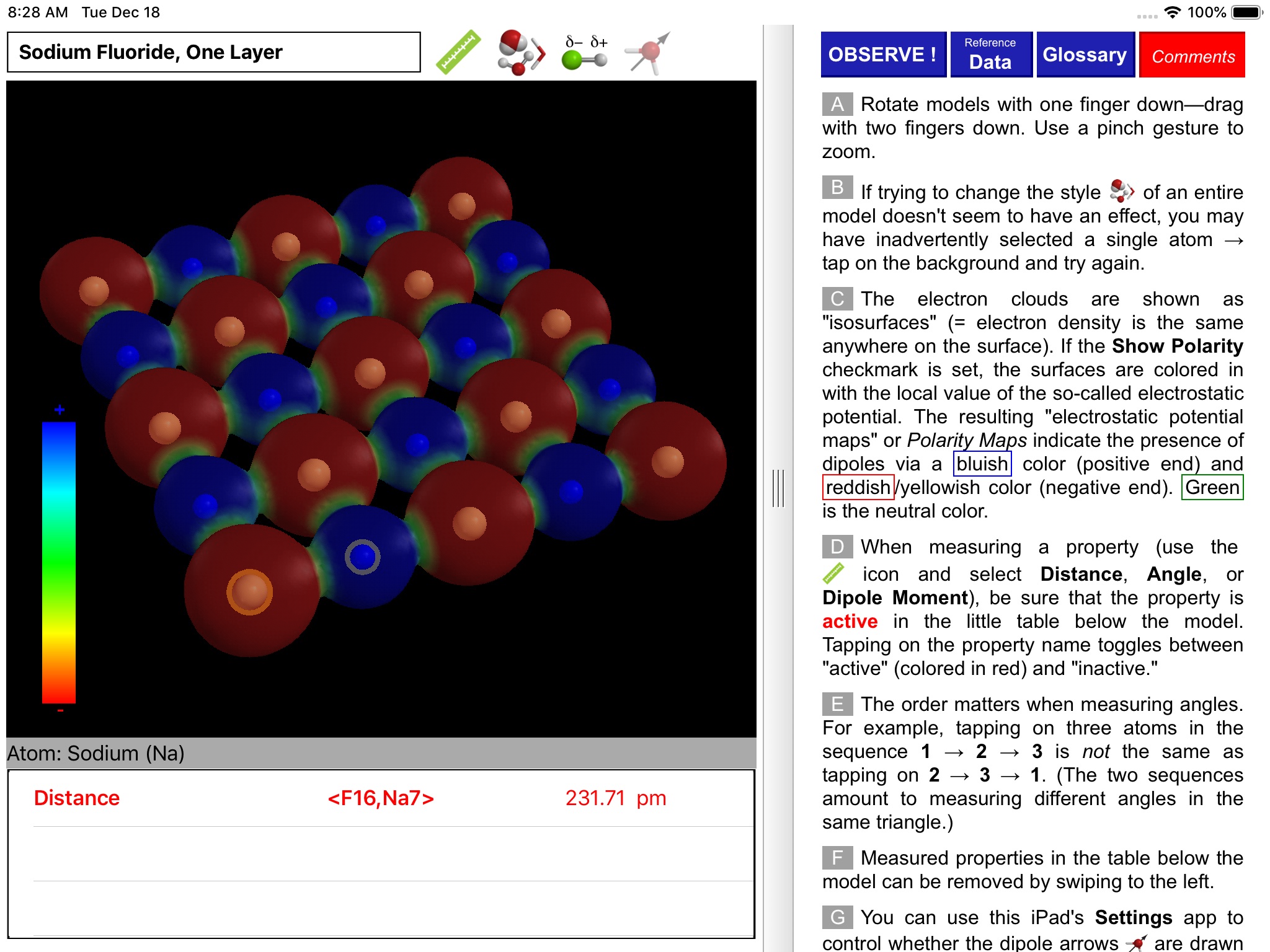
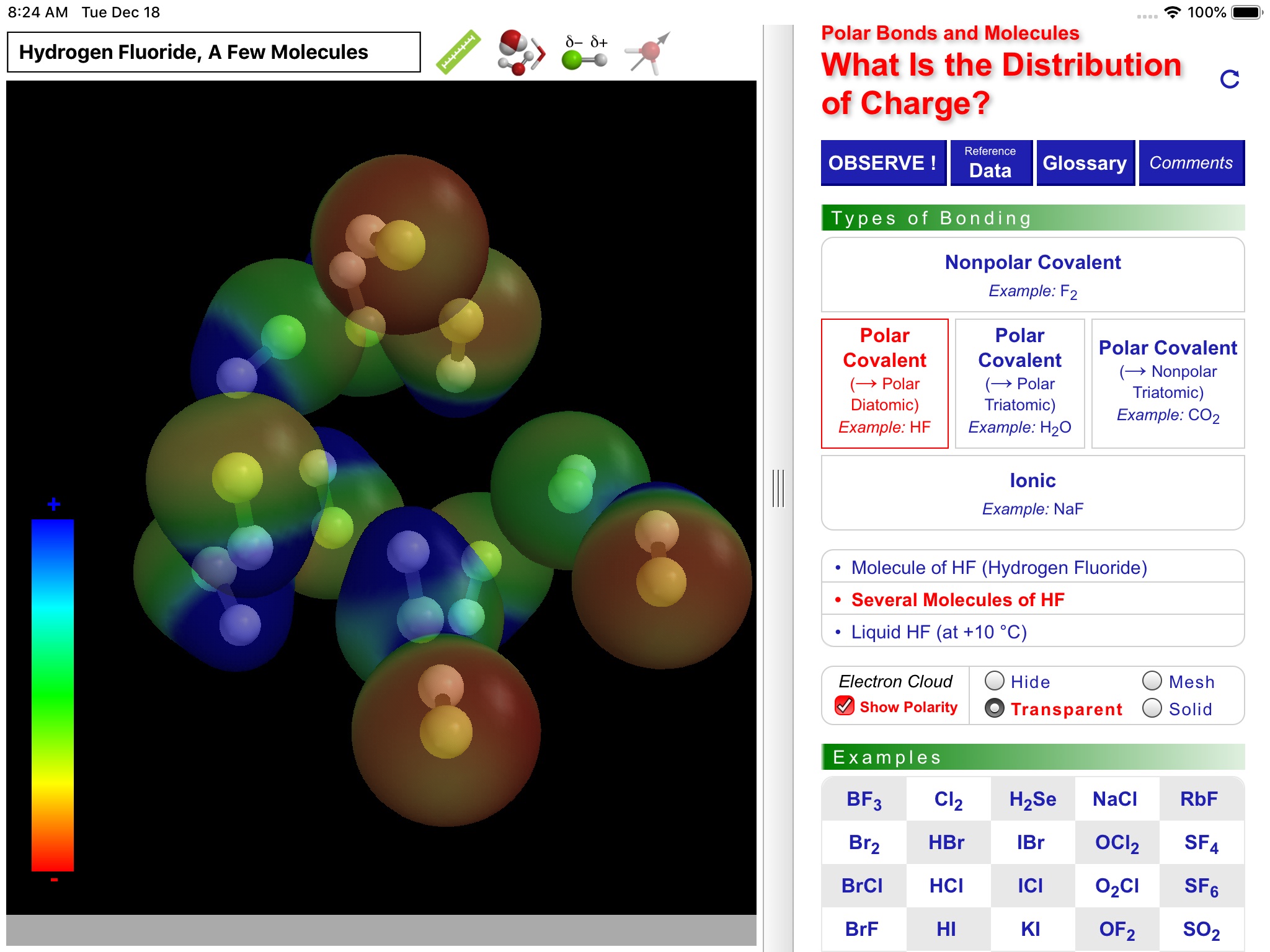
ODYSSEY Polar Bonds and Molecules takes an entirely visual approach to distinguishing between nonpolar, polar covalent, and ionic compounds. Quantum chemical calculations are employed to present electron density distributions for more than thirty systems. The distributions are also available as color-coded polarity maps (electrostatic potential maps). Pioneered by Wavefunction’s Spartan software and utilized in countless textbook illustrations, such maps provide an immediate and intuitive feel for the polarity and reactivity of compounds.
Using simple gestures, all models can be inspected at any orientation and zoom level. Different styles are available for the display of both molecules and electronic distributions. Bond distances and bond angles can be measured, the molecular dipole arrow can be shown, and a display of atomic charge labels can be requested. A glossary, comments section, and set of multiple-choice questions (with randomized options) are also available.
ODYSSEY Polar Bonds and Molecules helps in becoming familiar with the dichotomy of covalent and ionic bonding that is central to chemistry. The app also offers concrete examples to show how polar bonds in a molecule may (or may not) give rise to a compound that is polar.